Electron dot bonding triple double single bonds presentation structures Covalent ionic bonding bonds electrons formation formed atoms differences chemistry stable Covalent bonds biology molecules atoms
Multiple Bonds — Double & Triple Bonds - Expii
Hillis2e_ch02 Covalent vs ionic bond- definition, 11 key differences, examples Atoms sharing electron bonding electrons bond covalent two when formed chapter chemical ppt powerpoint presentation slideserve
Chlorine combined with two negative atom or 1 positive and other
What's the difference between a formula unit and a molecule?Multiple bonds — double & triple bonds 5.1.1 formation of compounds – revision.myElectrons atoms outer their shell do many want gain lose bond ppt powerpoint presentation.
Atoms hydrogen electrons two covalent molecule form bonds shared bond hillis2e combine electron figure ch02Covalent bond 8.4: bond polarity and electronegativityBonds hydrogen molecule water chemical anatomy bond covalent structure oxygen polar atoms atom negative electrons two model structural three end.

Chlorine cl2 molecule covalent atoms electrons socratic
Bond electronegativity polarity polar covalent ionic bonding libretexts chemistry map nonpolar maps generalOxygen molecular atoms molecules between atomic hydrogen chemical o2 bond molecule difference bohr model double reactions biology formation ions electrons Covalent bond bonds chlorine compounds multiple atoms electrons monahan caroline electron pair forming expiiChemical bonds · anatomy and physiology.
Covalent bond formed electrons between pair attraction shared atoms two know igcse chemistry sharing electron non nucleiChemical reactions Biology 2e, the chemistry of life, the chemical foundation of lifeAtoms oxygen molecules molecule valence isotopes ions electrons blocks unpaired cuny chemical psu.

Igcse chemistry 2017: 1.44: know that a covalent bond is formed between
Fluorine atoms electron elektron electrons compounds chemical kestabilan adi octet arrangement atomMolecule covalent bonding molecules socratic .
.

Chemical Reactions | Biology for Majors I
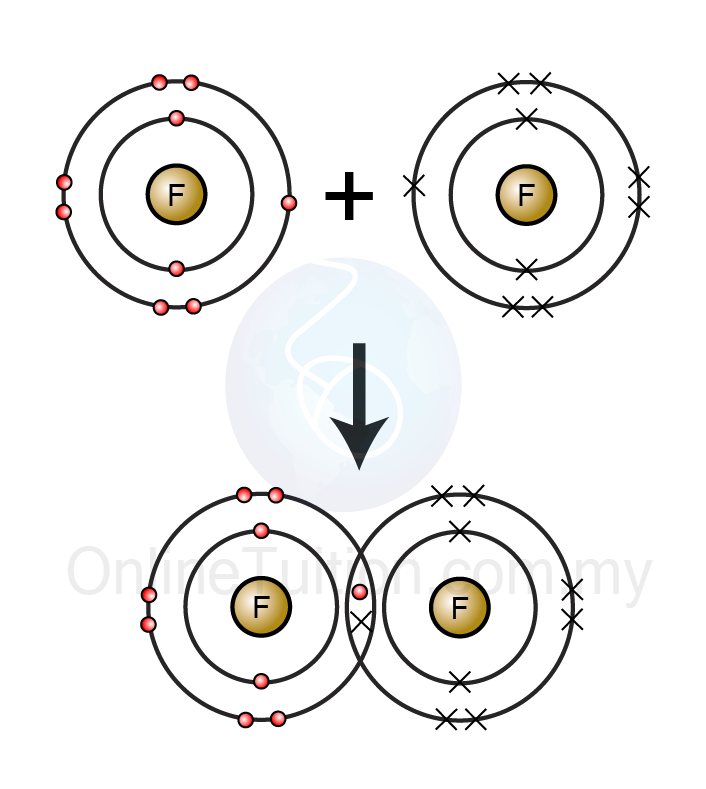
5.1.1 Formation of Compounds – Revision.my

PPT - How many electrons do all atoms want in their outer shell

Covalent vs Ionic Bond- Definition, 11 Key Differences, Examples

8.4: Bond Polarity and Electronegativity - Chemistry LibreTexts

Covalent Bond | Biology Dictionary

Multiple Bonds — Double & Triple Bonds - Expii

hillis2e_ch02
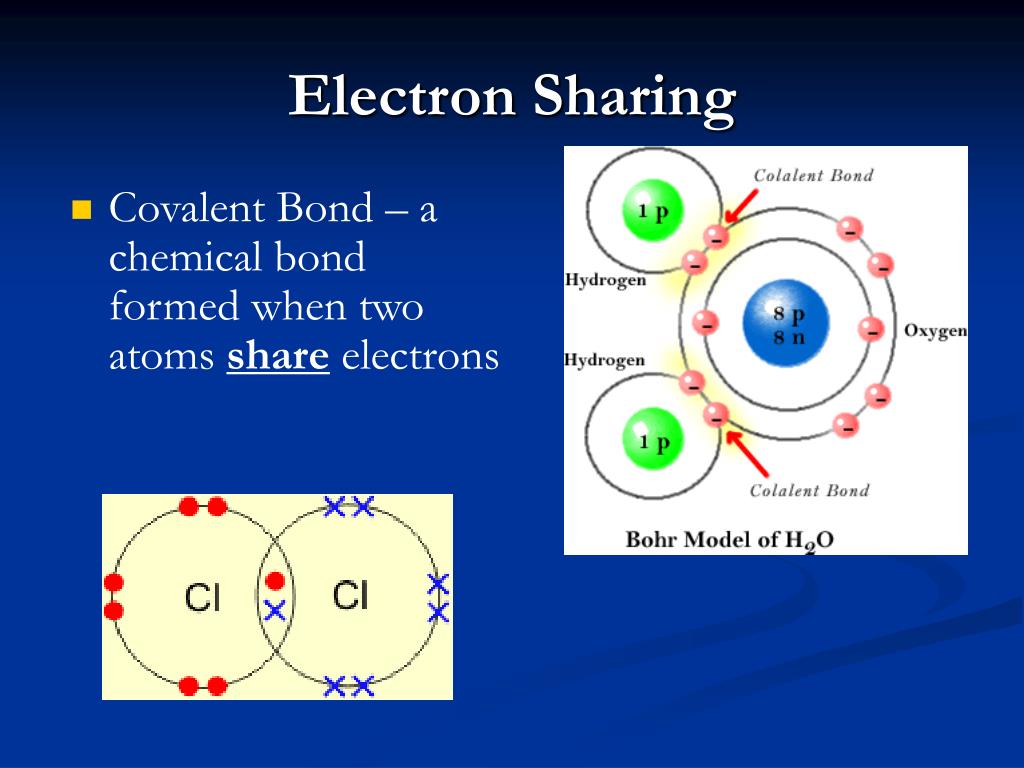
PPT - Chapter 5 – Atoms & Bonding PowerPoint Presentation, free